Added: Feb 02, 2026
Last edited: Feb 02, 2026
Recombinant Thrombin Market – Global Industry Analysis, Trends & Forecast (2026–2034)
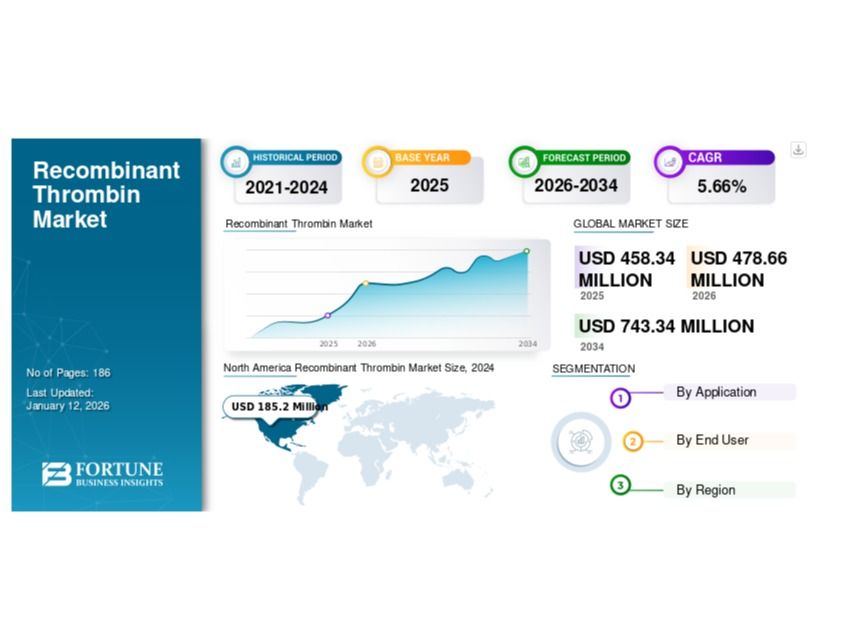
The global recombinant thrombin market is witnessing steady growth, driven by the rising number of surgical procedures, an expanding geriatric population, increasing cases of trauma and chronic diseases, and growing preference for safer hemostatic solutions. The market was valued at USD 458.34 million in 2025 and is projected to reach USD 743.34 million by 2034, registering a CAGR of 5.66% during the forecast period (2026–2034).
Understanding Recombinant Thrombin
Recombinant thrombin is a bioengineered enzyme that plays a vital role in blood coagulation by converting fibrinogen into fibrin. Produced using recombinant DNA technology, it serves as a safer alternative to human- or bovine-derived thrombin, significantly reducing the risks of immunogenic reactions, allergic responses, and disease transmission. This improved safety profile has led to growing adoption across surgical and clinical settings.
Download Free Sample: https://www.fortunebusinessinsights.com/enquiry/request-sample-pdf/recombinant-thrombin-market-113585
Market Drivers
Rising Volume of Surgical Procedures
The global increase in surgical interventions—particularly in cardiovascular, orthopedic, neurological, and general surgeries—is a major factor fueling market growth. Aging populations and the growing burden of chronic diseases continue to drive demand for effective bleeding control solutions during surgical procedures.
Shift Toward Safer Hemostatic Agents
Concerns over adverse immune reactions associated with animal- and plasma-derived thrombin have accelerated the shift toward recombinant alternatives. Healthcare providers increasingly favor recombinant thrombin due to its consistency, purity, and lower risk profile.
Advancements in Biotechnology
Continuous progress in biotechnology, along with increased awareness of patient safety, has supported the development and commercialization of recombinant thrombin products, further strengthening market expansion.
Market Restraints
High Product Cost
Recombinant thrombin products are relatively expensive due to complex manufacturing processes and strict regulatory requirements. These high costs may limit adoption, particularly in price-sensitive and developing markets.
Complex Manufacturing Processes
The production of recombinant thrombin involves sophisticated techniques such as gene cloning, fermentation, and purification, requiring advanced infrastructure and skilled expertise. These factors create barriers for new entrants and limit large-scale production.
Market Opportunities
Adverse effects associated with conventional thrombin products present strong growth opportunities for recombinant variants. With limited FDA-approved recombinant thrombin products currently available, there is significant potential for innovation, product development, and market entry by new players.
Key Market Trends
Product Innovation and Combination Therapies
Market participants are increasingly focusing on integrating recombinant thrombin into advanced hemostatic formulations and combination products. These innovations aim to improve ease of use, clinical efficiency, and patient outcomes during surgical procedures.
Market Segmentation
By Application
Therapeutic Applications dominate the market, owing to extensive use in surgical bleeding control.
Research Applications hold a notable share due to the widespread use of recombinant thrombin in molecular biology and protein research.
By End User
Hospitals and Ambulatory Surgery Centers (ASCs) represent the largest end-user segment, supported by high surgical volumes.
Research Institutes are experiencing growing demand as life sciences and biotechnology research expands globally.
Regional Outlook
North America holds the largest market share, accounting for 42.24% in 2025, driven by advanced healthcare infrastructure, high surgical adoption, and early uptake of recombinant technologies.
Europe maintains a strong position due to an aging population and increasing number of surgical procedures.
Asia Pacific is the fastest-growing region, supported by rising healthcare investments, improving medical infrastructure, and a growing patient base.
Latin America and the Middle East & Africa are emerging markets, benefiting from gradual improvements in healthcare facilities and increasing awareness of advanced hemostatic solutions.
Competitive Landscape
The recombinant thrombin market is semi-fragmented, with several established players focusing on research, product innovation, and strategic expansion. Key companies operating in the market include:
Baxter
Merck KGaA
Bio-Techne
AMSBIO
Enzyme Research Laboratories
Proteintech Group
Leading companies are investing heavily in research and development, expanding manufacturing capabilities, and strengthening their global presence to gain a competitive edge.
Source: https://www.fortunebusinessinsights.com/recombinant-thrombin-market-113585
Conclusion
The recombinant thrombin market is expected to experience consistent growth through 2034, supported by rising surgical demand, advancements in biotechnology, and increasing preference for safer hemostatic agents. While high costs and manufacturing complexities remain challenges, ongoing innovation and expanding healthcare access in emerging economies are likely to shape a positive long-term outlook for the market.

